Libtayo Demonstrates 68% Risk Reduction in High-Risk CS Cell Carcinoma

31 May 2025
The new data from the Phase 3 C-POST trial presented at ASCO 2025 demonstrated that cemiplimab (Libtayo) reduced the risk of recurrence or death by 68% in high-risk cutaneous squamous cell carcinoma. Two-year disease-free survival (DFS) was 87% with cemiplimab versus 64% with placebo. Locoregional and distant recurrence risks were reduced by 80% and 65%, respectively.
Regeneron Pharmaceuticals unveiled compelling data from the Phase 3 C-POST trial, positioning cemiplimab (Libtayo) as a potentially practice-changing option in the adjuvant setting for patients with high-risk cutaneous squamous cell carcinoma (CSCC). Detailed results were presented in an oral session at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting and simultaneously published in the NEJM.
“The Phase 3 C-POST trial demonstrates that cemiplimab is a highly active therapy in high-risk CSCC, with clinically meaningful outcomes across primary and secondary endpoints and exceptionally low rates of locoregional and distant recurrence,” said Dr. Danny Rischin, lead investigator of the trial.
A Meaningful Impact on Recurrence
The C-POST trial, a randomized, placebo-controlled, double-blind study, enrolled 415 patients with nodal or non-nodal high-risk features, including extracapsular extension, multiple involved lymph nodes, or T4 lesions.
After a median follow-up of 24 months, cemiplimab demonstrated a 68% reduction in the risk of disease recurrence or death compared to placebo (HR: 0.32; 95% CI: 0.20–0.51; p<0.0001). Notably, median disease-free survival (DFS) was not reached for patients receiving cemiplimab, while it was 49 months for those on placebo. At the two-year mark, DFS rates stood at 87% with Libtayo versus 64% with placebo.
Beyond the primary endpoint, secondary outcomes further underscore cemiplimab’s clinical benefit. The trial reported an 80% reduction in the risk of locoregional recurrence (HR: 0.20; 95% CI: 0.09–0.40) and a 65% reduction in the risk of distant recurrence (HR: 0.35; 95% CI: 0.17–0.72). Updated overall survival (OS) data, with six months of additional follow-up, suggest an emerging survival benefit for cemiplimab(HR: 0.78; 95% CI: 0.39–1.56).
Effectiveness Across PD-L1 Expression Levels
An exploratory analysis of the data revealed that cemiplimab’s efficacy was consistent regardless of PD-L1 expression. In patients with PD-L1 expression ≥1%, cemiplimab reduced the risk of disease recurrence or death by 72% (HR: 0.28; 95% CI: 0.15–0.52). In those with PD-L1 <1%, the risk reduction was 68% (HR: 0.32; 95% CI: 0.12–0.86), indicating broad applicability of this approach across biomarker subgroups.
Implications for Clinical Practice
“These results show the continued promise of Libtayo in non-melanoma skin cancers,” said Dr. Israel Lowy, Clinical Development Unit Head, Oncology, at Regeneron. “Libtayo is the first medicine to demonstrate a statistically significant benefit in patients who have high-risk features for recurrence after resection of CSCC and has the potential to become a new standard of care in the adjuvant setting. We are working with global regulatory authorities to bring this new option to patients as quickly as possible.”
Safety Profile
Safety findings were consistent with previous studies of cemiplimab in other tumor types. Adverse events (AEs) of any grade were reported in 91% of patients in the cemiplimab arm and 89% in the placebo arm. Grade ≥3 AEs occurred in 24% and 14% of patients, respectively. Common AEs in the cemiplimab arm included fatigue, pruritus, rash, diarrhea, arthralgia, and hypothyroidism. Hypertension was the only grade ≥3 AE occurring in more than 2% of patients. Discontinuation due to AEs occurred in 10% of patients receiving cemiplimab, compared to 2% on placebo.
Regulatory applications for Libtayo’s use in adjuvant CSCC have been submitted in the United States and European Union, signaling a potential shift in the treatment paradigm for this challenging disease.
About Cemiplimab (Libtayo)
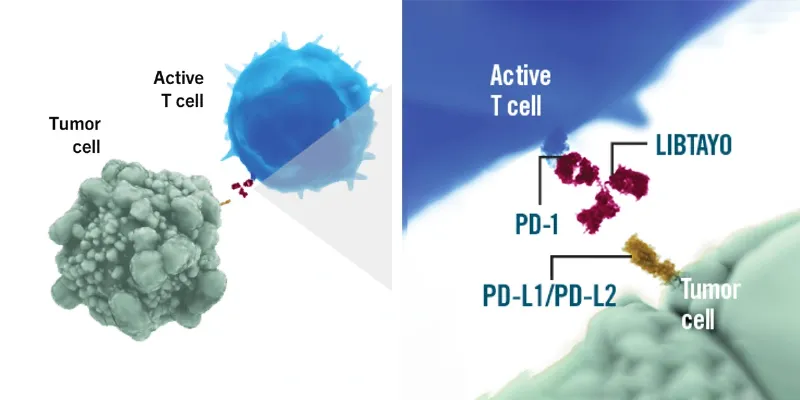
Cemiplimab (Libtayo) is a fully human monoclonal antibody targeting the immune checkpoint receptor PD-1 on T cells and was invented using Regeneron's proprietary VelocImmune® technology. By binding to PD-1, Libtayo has been shown to block cancer cells from using the PD-1 pathway to suppress T-cell activation. Libtayo has been approved by regulatory authorities in more than 30 countries in one or more indications, including for certain adult patients with advanced basal cell carcinoma (BCC), advanced CSCC, advanced non-small cell lung cancer (NSCLC) and advanced cervical cancer. The extensive clinical program for Libtayo is focused on difficult-to-treat cancers. Libtayo is currently being investigated in trials as a monotherapy, as well as in combination with either conventional or novel therapeutic approaches for other solid tumors and blood cancers.











Comments
No Comments Yet!