Bacteria Cloak Viruses to Safely Deliver Cancer-Killing Payloads into Solid Tumors
16 August 2025
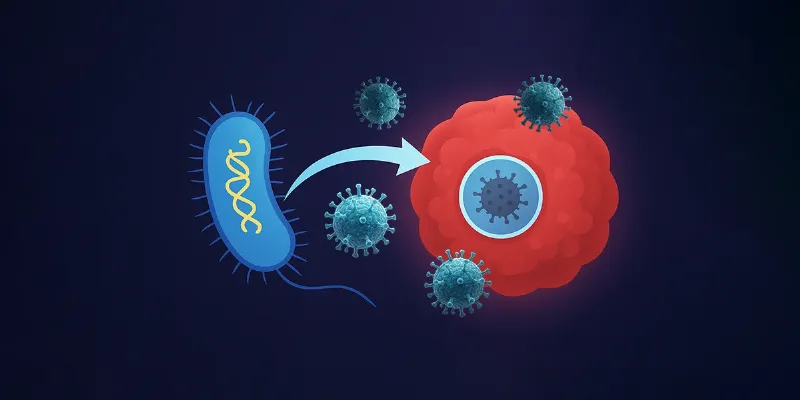
Researchers at Columbia Engineering have developed a novel cancer therapy using Salmonella typhimurium to deliver an oncolytic virus directly into tumors, evading immune detection. The platform, called CAPPSID (Coordinated Activity of Prokaryote and Picornavirus for Safe Intracellular Delivery), employs tumor-targeting bacteria as microscopic couriers to transport the virus past immune defenses and deep into solid tumors. This breakthrough may enable safer, more targeted solid tumor immunotherapies.
The concept, detailed in Nature Biomedical Engineering, marks a first in the field: a synthetically engineered cooperation between prokaryotes and viruses, tailored for safe and targeted tumor destruction.
“We programmed the bacteria to act as a Trojan horse by shuttling the viral RNA into tumors and then lyse themselves directly inside of cancer cells to release the viral genome, which could then spread between cancer cells,” says Zakary Singer, co-lead author and synthetic biologist.
Engineering Microbial Collaboration
The immune system’s swift response to foreign invaders—particularly viruses—has long challenged the clinical utility of oncolytic virotherapy. Patients often harbor pre-existing antibodies that neutralize viral agents before they reach their tumor targets. The Columbia team, led by biomedical engineer Dr. Tal Danino and in collaboration with virologist Dr. Charles M. Rice of Rockefeller University, devised a workaround: hide the virus inside bacteria.
Specifically, Salmonella typhimurium, a strain known for its natural affinity for the low-oxygen, nutrient-rich microenvironment of tumors, was genetically engineered to carry the RNA genome of Senecavirus A—a picornavirus with known cancer-killing potential. Once inside the tumor, the bacteria infiltrate cancer cells and self-destruct, releasing the viral payload where it can replicate and spread exclusively within the tumor tissue.
“We aimed to enhance bacterial cancer therapy by enabling the bacteria to deliver and activate a therapeutic virus directly inside tumor cells, while engineering safeguards to limit viral spread outside the tumor,” says co-lead author Dr. Jonathan Pabón, PhD candidate at Columbia Engineering. “Our system demonstrates that bacteria can potentially be used to launch an oncolytic virus to treat solid tumors in patients who have developed immunity to these viruses.”
Tumor-Selective Activation, Immune Evasion
CAPPSID’s innovation lies not just in delivery, but also in control. The virus is genetically altered to be dependent on a bacterial protease for maturation—a protease that is produced only by the engineered bacteria. This synthetic interdependence ensures that mature, infectious virions can only form in the immediate vicinity of the bacteria—i.e., inside tumors.
“By bridging bacterial engineering with synthetic virology, our goal is to open a path toward multi-organism therapies that can accomplish far more than any single microbe could achieve alone,” says Singer. “The bacteria act as an invisibility cloak, hiding the virus from circulating antibodies, and ferrying the virus to where it is needed.”
By encapsulating the viral RNA within bacteria, the team also bypasses circulating antiviral antibodies—a major barrier in patients with prior virus exposure. In preclinical mouse models, the system succeeded in launching potent intratumoral infections even in animals with active antiviral immunity.
A Toolkit for Next-Generation Living Medicines
On their own, engineered bacteria and oncolytic viruses have faced limitations: poor tumor penetration, immune clearance, and safety concerns. CAPPSID addresses all three.
“Spreadable viral particles could only form in the vicinity of bacteria, which are needed to provide special machinery essential for viral maturation in the engineered virus, providing a synthetic dependence between microbes,” Singer says. That safeguard adds a second layer of control: even if the virus escapes the tumor, it won’t spread in healthy tissue.
The researchers envision CAPPSID as a modular toolkit that could one day be adapted to a range of tumor types and payloads. Ongoing efforts include testing additional viral genomes, evaluating CAPPSID across various tumor models, and pairing it with clinically validated bacterial strains.
Importantly, the team has filed a U.S. patent (WO2024254419A2) and is initiating translational steps toward first-in-human studies.











Comments
No Comments Yet!