Redesigned CD40 Antibody Triggers Systemic Tumor Regression and Complete Remission
15 August 2025
A first-in-human phase 1 trial of the Fc-enhanced CD40 agonist antibody 2141-V11 has shown striking results: six of 12 patients with advanced cancers experienced tumor shrinkage, including two complete remissions in melanoma and breast cancer. Delivered intratumorally, the drug triggered systemic immune responses, induced tertiary lymphoid structures, and achieved efficacy without dose-limiting toxicities.
For two decades, CD40 agonist antibodies were seen as potential game-changers in oncology. In theory, they could turn cold tumors hot—rallying the immune system against cancer. In practice, they disappointed: early clinical trials revealed little efficacy and alarming toxicities, including cytokine storms, thrombocytopenia, and liver damage. The promise of CD40 agonism seemed destined for the scrap heap of failed immunotherapies.
Now, results from a first-in-human phase 1 trial published in Cancer Cell suggest that a re-engineered version of this drug class may finally deliver on its long-elusive potential. The Fc-enhanced antibody, called 2141-V11, not only triggered local immune responses when injected directly into tumors but also unleashed systemic tumor regression—including complete remission in two patients with metastatic disease.
“Seeing these significant shrinkages and even complete remission in such a small subset of patients is quite remarkable,” says Juan Osorio, first author of the study and medical oncologist at Memorial Sloan Kettering Cancer Center.
A New Spin on a Stalled Strategy
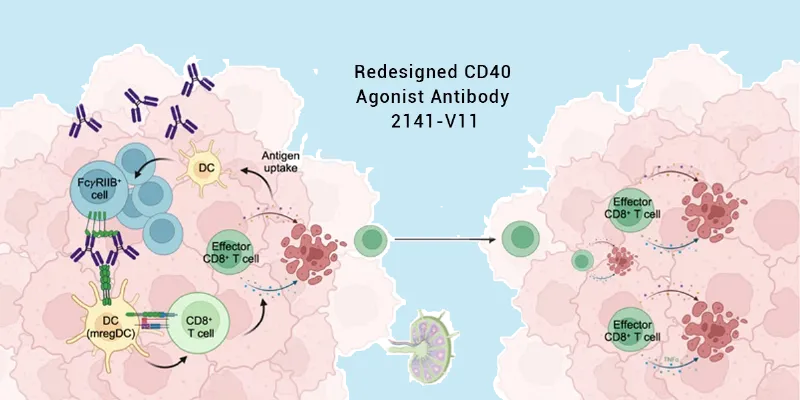
CD40 is a cell-surface receptor belonging to the TNF receptor superfamily, primarily expressed on immune cells. When activated, it primes dendritic cells, enhances T-cell responses, and helps generate durable antitumor immunity.
Earlier drug designs sought to activate CD40 broadly through intravenous infusion. But because CD40 is expressed throughout the body, this approach caused widespread immune activation and toxic side effects.
Jeffrey V. Ravetch’s lab at Rockefeller University tackled the problem from two angles. First, they redesigned the antibody to enhance binding to the inhibitory FcγRIIB receptor, improving crosslinking and boosting potency by a factor of ten. Second, they changed the route of delivery: rather than intravenous dosing, they injected the drug directly into tumors, minimizing systemic exposure.
A Surprising Systemic Ripple
In the phase 1 trial (NCT04059588), 12 patients with advanced, treatment-resistant cancers—including melanoma, renal cell carcinoma, and breast cancer—received intratumoral injections of 2141-V11. The results were striking:
- No dose-limiting toxicities were observed.
- Six patients experienced tumor shrinkage, and in two cases—metastatic melanoma and breast cancer—tumors disappeared completely.
- Crucially, the drug induced abscopal effects: tumors outside the injection site also regressed or vanished.
One patient with metastatic melanoma had dozens of lesions across her leg and foot. Only a single tumor on her thigh was injected. After repeated dosing, all lesions disappeared. A similar phenomenon occurred in a breast cancer patient with metastases in the skin, liver, and lungs, where injecting just a skin lesion led to clearance of all tumors.
“This effect—where you inject locally but see a systemic response—that’s not something seen very often in any clinical treatment,” Ravetch notes. “It’s another very dramatic and unexpected result from our trial.”
Building Immune “Lymph Nodes” Inside Tumors
Tissue analysis provided an explanation. Tumors treated with 2141-V11 became infiltrated with immune cells—dendritic cells, T cells, and B cells—that organized into tertiary lymphoid structures (TLS). These structures resemble miniature lymph nodes and are strongly associated with favorable responses to immunotherapy.
Notably, TLS were also detected in tumors that were never injected, suggesting that the drug not only primes local immunity but also instructs systemic immune surveillance.
In mouse models engineered to express human CD40 and Fcγ receptors, intratumoral 2141-V11 likewise induced TLS formation, robust CD8+ T-cell effector activity, and long-term immune memory—even without lymph node priming. This independence from conventional immune hubs hints at a new way of generating systemic immunity directly within tumor beds.
Toward Precision Immunotherapy
Future trials are now underway at Memorial Sloan Kettering and Duke University, testing 2141-V11 in bladder, prostate, and brain cancers. With nearly 200 patients enrolled across early-phase studies, researchers hope to refine predictors of response and explore combination strategies that may convert non-responders into responders.
As Osorio notes, “As a general rule, only 25 to 30% of patients will respond to immunotherapy, so the biggest challenge in the field is to try to determine which patients will benefit from it. What are the indicators or predictors of response? And how can we convert non-responders into responders?”
The emergence of 2141-V11 offers a fresh take on an old idea: that careful engineering of both antibody structure and delivery route can rescue promising but abandoned drug classes. If larger trials confirm the early signals—safety, systemic responses, and durable remissions—CD40 agonists may yet carve a niche in the immunotherapy arsenal.











Comments
No Comments Yet!